Powering Technology With Photosynthetic Microbes
Plant-like animals, microbial symbiosis, and the reciprocity between technology and nature
Elysia chlorotica, a small sea snail that lives in shallow waters along the reefs of Atlantic coasts, can sustain itself for several months by drawing the energy it needs directly from sunlight. But, unlike a plant, it is not able to produce energy entirely on its own; to be precise, it would be appropriate to say that it steals it. In its early years, the snail feeds on an alga called Vaucheria litorea, piercing its cells with an organ evolved specifically for this purpose. By sucking the algae's contents, it extracts its chloroplasts, the tiny internal structures responsible for photosynthesis. These stolen organelles, appropriately called “kleptoplasts,” are absorbed and integrated into the snail's own cells, where they continue to function as if nothing had changed.
It is a noteworthy phenomenon: in plants and algae, chloroplasts are not autonomous but act thanks to thousands of proteins encoded in the nucleus of their host cell. Elysia chlorotica, however, operates them in the complete absence of this support system, and biologists do not know how this is possible. According to some, the snail may have borrowed some genes from Vaucheria litorea in the course of evolution through a process called “horizontal gene transfer,” which would make it a kind of genetic chimera somewhere between animal and alga. Others think that Elysia chlorotica reprogrammed its own proteins to keep the stolen organelles “alive,” if life can be spoken of (to explore this question further, I refer you to a fascinating article recently published in Asimov press). In any case, the result is a living hybrid: a soft-bodied solar panel, oddly resembling a leaf, that feeds on light as a plant would.
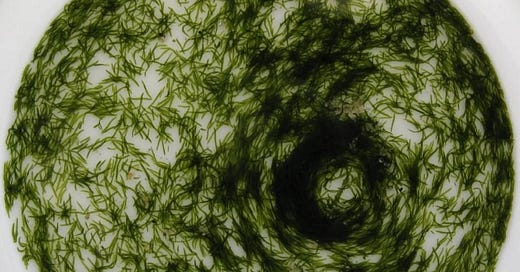
Symsagittifera roscoffensis, also improperly but evocatively called a “plant-animal” worm, pushes this hybridization one step further: its entire life depends on an alga that settles into its cells shortly after birth. In this unexpected alliance, the alga photosynthesizes, energizing the animal; the worm moves toward the light, granting the alga as much sun exposure as possible. These worms live on shorelines in communities of thousands of individuals, and form teeming mats that, when dense enough, synchronize their movements into circular whirpools. The reason for this spiral dance is not entirely clear. According to some scholars, it helps maximize the worm community's surface exposure to light, in a kind of liquid architecture—an ephemeral superorganism held together by the unlikely meeting of enormously distant worlds.
More than individual organisms, Elysia chlorotica and Symsagittifera roscoffensis are strange, moving gardens that transplant and cultivate other creatures within their own skin. These beings thrive as “holobionts”: intimate communities of different species that exchange metabolic products, survive and evolve as one organism. The concept of “holobiont” was conceived in its modern sense by biologist Lynn Margulis, who was the first scientist to recognize the fundamental importance of symbiosis-the mutually beneficial coexistence of different species-for the evolution of life on Earth. It was Margulis who recognized that all eukaryotic organisms on the planet are heirs to an ancient symbiotic encounter between different microorganisms that have learned to coexist in a single body. In recent years, it has become increasingly clear to scientists that holobionts, not all as conspicuous as those I have just described, are not exceptions but rather the rule of complex life on our planet. Virtually all multicellular organisms on Earth can be considered holobionts: from cows that digest grass with the help of specialized gut microbes, to corals that build extensive structures in cooperation with photosynthetic algae, to plants that coexist with nitrogen-fixing bacteria in their roots, to humans.
Each of us carries trillions of microbes in our gut, on our skin and even in our lungs, a community of invisible organisms whose numbers (as we now often hear) equal or exceed the number of cells in our bodies. The genes of these microbial partners, together with those of the host organism, form a shared genetic system called the “hologenome,” with extraordinary adaptive capabilities. Faced with environmental change, in fact, a holobiont does not have to wait for slow genetic mutations to occur in the host organism's DNA. The microbiome can change much more rapidly, borrowing from the countless adaptations that microbes, in their very long evolutionary history, learned long before we did. These changes can modulate many functions, such as metabolism, the immune system and even behavior, sometimes within a single generation. Microbes are radical innovators: they accelerate species adaptation and trigger profound changes that unfold from the molecular to the planetary scale. These capacities for innovation and adaptation should give us food for thought. How might microbes change the way we imagine and build our technologies? What might it mean in practice to build technologies as holobionts?
A few months ago, a coalition of scientists from different disciplines issued a call to action, urging governments, industries and institutions to develop and adopt microbial technologies as a key strategy to mitigate the climate crisis. Published simultaneously in leading scientific journals, the call argues that microbes, long neglected in ecological policies, can perform essential functions: sequestering carbon, producing energy, detoxifying polluted soils, to mention a few. If microbes help living systems develop better and more sustainable metabolisms, could they also help technological systems do the same?
One striking example of this possibility dates back to 2022, when a group of researchers succeeded in powering a microprocessor not with a battery or solar panel, but with photosynthetic microorganisms. The technology in question, called a “biophotovoltaic cell”, is a small laboratory device about the size of an AA battery that uses the cyanobacterium Synechocystis to generate a constant electric current. Through its own metabolic activity, the cyanobacteria culture kept an Arm Cortex M0+ chip running for more than six months under ambient light-only conditions. Like Elysia chlorotica or Symsagittifera roscoffensis, this algae-powered computer also uses the metabolism of a microscopic creature to power a much larger and more complex structure. We might consider it, with all due caution, a “technological holobiont”: a hybrid entity that derives its functionality from an unlikely and fruitful encounter with another organism.
When the researchers first set up the device, they inoculated it with a single species of photosynthetic bacteria. But over time, as the experiment took place in open contact with a non-sterile space, other microbes from the air and surroundings began to occupy the biophotovoltaic cell. Eventually, the anode (the part of the device where electrons are collected) was colonized by a complex microbial community hosting several types of bacteria, some of which were electroactive, i.e., capable of transferring electrons directly to the anode, and others that probably played supporting roles in the battery's “metabolic ecosystem”.
Rather than leading to a loss of efficiency, this spontaneous microbial contamination proved surprisingly beneficial. The system remained stable and functional for months, with no sign of energy decline. The study authors believe that the presence of a microbial consortium, rather than a single strain of cyanobacteria, made the system more resilient and adaptable, especially under unstable light and temperature conditions. “Evidence was found for a complex biome containing a wide range of microorganisms,” the researchers write. “Having a consortium of microorganisms in the anode compartment, with photoautotrophs and heterotrophs sharing different functions, may offer enhanced stability and less susceptibility to contamination.” Not unlike holobionts in nature, each new structure, whether artificial or organic, is a niche that can accommodate unforeseen complexities.
Architect Rachel Armstrong, a longtime pioneer in devising hybrid systems that straddle biology and technology, has proposed integrating a biophotovoltaic system similar to the one I have just described by embedding it in human lived environments. According to Armstrong, we should aspire to build an “engineered symbiosis with microbes,” creatures we tend to view as harmful invaders but are instead potential allies, “among the most powerful world-shaping forces we know.” Her Living Architecture project, presented at the 2016 Architecture Biennale, proposed the construction of biophotovoltaic cells integrated within the structure of buildings. These “living bricks” would be able to feed on domestic wastewater and, through photosynthesis, produce enough energy to power digital home systems capable of communicating and self-regulating without any human intervention, participating in the so-called “Internet of Things.”

Technologies such as these are still a long way from being adopted on a large scale, but, rather than as concrete solutions, they interest me as the first manifestations of a new way of thinking about technology: a paradigm that is a profound departure from the rigid, closed, and pre-programmed machines that preceded them. One of the most interesting features of technologies of this kind is the way they manage the energy they need: rather than depending on external energy infrastructure, they are able to harvest it from their environment and manage it autonomously on a molecular scale. In a paper I recently published, I called this feature “micro-sustainability,” proposing the idea that a radical rethinking of the (currently unsustainable) metabolism of our technologies must start at the microscopic scale of the materials from which they are built. In designing a micro-sustainable technology, we have much to learn from microbes and the communities they build with the more complex organisms that host them.
I often reflect on the ability of technologies to act as mirrors: the more I analyze and study them, the more I realize that the engineering models we apply to our artifacts reflect the image we have of ourselves, of life in general, and of the world as a whole. Unlike a literal mirror, however, both sides—the primary reality and its reflection—are linked by an inseparable reciprocity. Building technologies as holobionts is one of the many ways in which this reciprocity is currently manifesting. Our recognition of the importance of microbial symbiosis in the evolution of life on Earth opens up the possibility of building more sustainable technologies; in turn, building these technologies allows us to construct a more cooperative relationship with the more-than-human world. As our view of nature changes, our technologies transform. As our technologies transform, our view of the world, and the way we act within it, may—eventually—also change.
Bibliography
Armstrong, Rachel. “Biodesign for a Culture of Life: Of Microbes, Ethics, and Design,” 2022. https://doi.org/10.21606/drs.2022.144.
Bombelli, P., A. Savanth, A. Scarampi, S. J. L. Rowden, D. H. Green, A. Erbe, E. Årstøl, et al. “Powering a Microprocessor by Photosynthesis.” Energy & Environmental Science 15, no. 6 (2022): 2529–36. https://doi.org/10.1039/D2EE00233G.
Chitayat, Liyam. “Mitochondria Are Alive.” Asimov Press, November 6, 2024. https://doi.org/10.62211/38pe-75hu.
Franks, Nigel R., Alan Worley, Katherine A. J. Grant, Alice R. Gorman, Victoria Vizard, Harriet Plackett, Carolina Doran, Margaret L. Gamble, Martin C. Stumpe, and Ana B. Sendova-Franks. “Social Behaviour and Collective Motion in Plant-Animal Worms.” Proceedings of the Royal Society B: Biological Sciences 283, no. 1825 (February 24, 2016): 20152946. https://doi.org/10.1098/rspb.2015.2946.
Peixoto, Raquel, Christian R. Voolstra, Lisa Y. Stein, Philip Hugenholtz, Joana Falcao Salles, Shady A. Amin, Max Häggblom, et al. “Microbial Solutions Must Be Deployed against Climate Catastrophe.” Nature Communications 15, no. 1 (November 11, 2024): 9637. https://doi.org/10.1038/s41467-024-53680-w.
Rosenberg, Eugene, and Ilana Zilber-Rosenberg. “The Hologenome Concept of Evolution after 10 Years.” Microbiome 6, no. 1 (December 2018): 78. https://doi.org/10.1186/s40168-018-0457-9.
Rumpho, Mary E., Elizabeth J. Summer, Brian J. Green, Theodore C. Fox, and James R. Manhart. “Mollusc/Algal Chloroplast Symbiosis: How Can Isolated Chloroplasts Continue to Function for Months in the Cytosol of a Sea Slug in the Absence of an Algal Nucleus?” Zoology 104, no. 3–4 (January 2001): 303–12. https://doi.org/10.1078/0944-2006-00036.
Tripaldi, Laura. “Softness: An Ecological Paradigm for Embodied Technological Intelligence.” Social Epistemology 39, no. 3 (May 4, 2025): 322–31. https://doi.org/10.1080/02691728.2025.2449607.





Hi Laura,
I just came across this entry and felt compelled to reach out. I’m currently collaborating with Paolo Bombelli, Joshua Lawrence, and Alberto Scarampi—the authors of the paper Powering a Microprocessor by Photosynthesis—on a project that builds on their research.
Together, we are developing a musical instrument that generates compositions from environmental light, using the photosynthetic activity of cyanobacteria. I am contributing to this collaboration as visual and sound artist, exploring ways to translate the biochemical processes into sensory experiences.
We co-published a related research paper in December last year, which I’d be happy to share with you.
https://www.tandfonline.com/doi/full/10.1080/26388081.2024.2434476
If you're interested, I’d love to exchange thoughts and stay in touch.
kind regards, Lena
Loved this piece all the way through, and really hopeful (for technology’s sake and our own) in the turn of language you mention—in shifting away from mechanical or computer narratives to understand humans, and thinking of our technologies as organisms and assemblages. Thanks for writing!